Covid-19 Vaccine (vero Cell) Inactivated, Vero Cell News Articles Etc European Pharmaceutical Review
It is a preparation made from a novel coronavirus strain CZ02 grown in the kidney cell cultures Vero Cell of the African green monkey and contains inactivated. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant.
Uruguay Coronavac Is 66 Effective In Preventing Covid 19
The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune.
Covid-19 vaccine (vero cell) inactivated. The citys leaders have said. When a person is given the. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date.
This trial is a randomized study to evaluate the immunogenicity of COVID-19 Vaccine Vero Cell Inactivatedand in population aged 60 years old and. Inactivated vaccines for Covid-19 in the pipeline. BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or BIBP vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharm s Beijing Institute.
COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19. COVID-19 Vaccine Vero Cell Inactivated Brief Edition Created Date.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population. The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys. There is an urgent demand of a vaccine to control COVID-19 disease and SARS-CoV-2 dissemination worldwide.
A SARS-CoV-2 inactivated vaccine has been developed in Institute of Medical Biology Chinese Academy of Medical Sciences. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 is responsible for the coronavirus disease 2019 COVID-19 pandemic that emerged in December 2019 in Wuhan.
COVID-1 Vaccine Vero Cell Inactivated Sinopharm COVID-19 Vaccine Explainer 24 MAY 2021 Stability and storage Vaccine storage temperature Store in the. COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. Its easy storage requirements make it highly suitable for low-resource settings.
A Study to Evaluate the Efficacy Safety and Immunogenicity of SARS-CoV-2 Vaccine Vero Cells Inactivated in Healthy Adults Aged 18 Years and Older. The SARS-CoV-2 inactivated vaccine will. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements.
The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements.
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex
500 000 Doses Of Sinopharm Covid 19 Vaccine Arrive In Vietnam Today
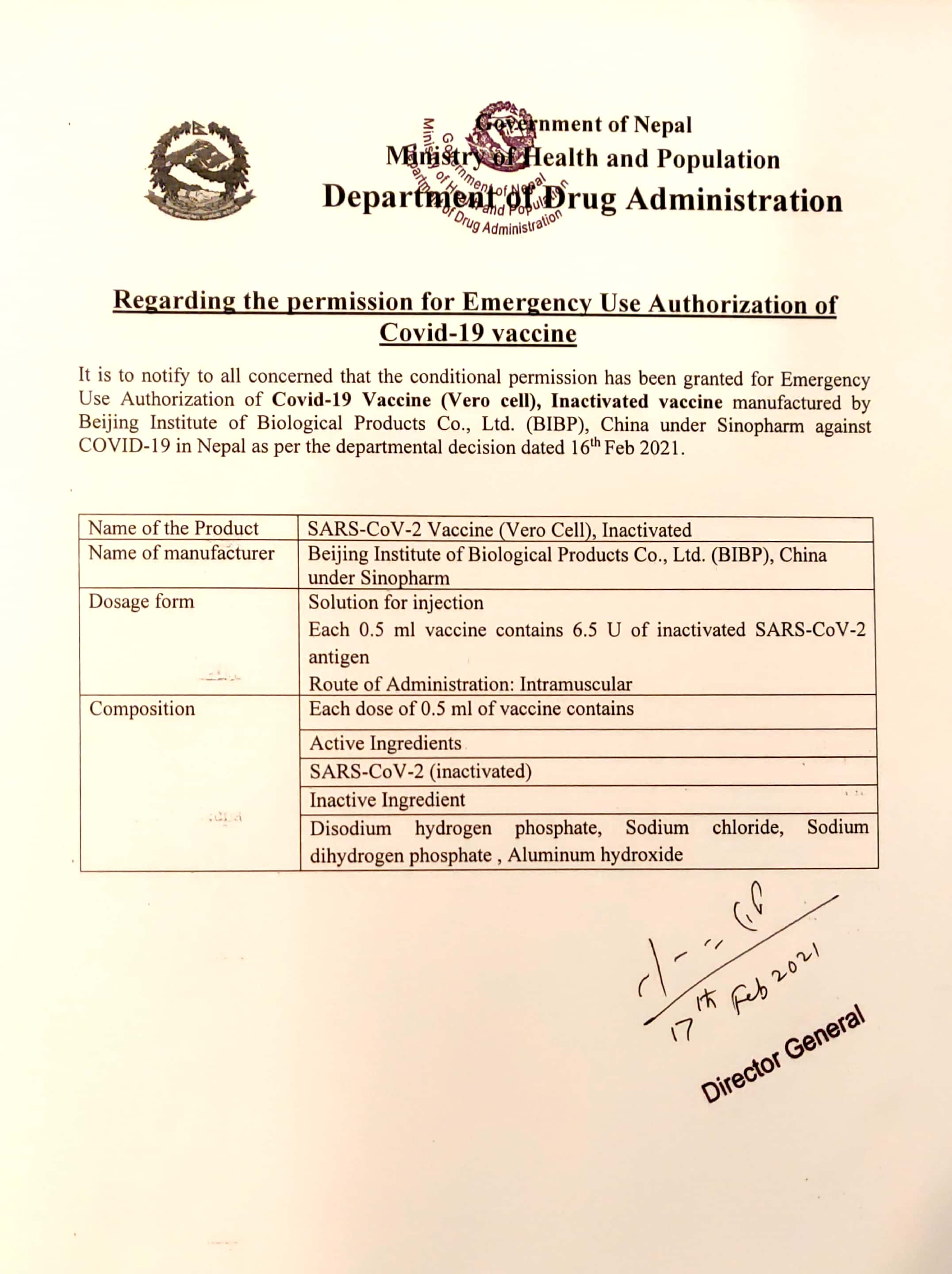
Dda Eua Of Covid 19 Vaccine Vero Cell Inactivated Manufactured By Bibp Under Sinopharm

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Who Approves Sinovac Covid 19 Vaccine For Emergency Use Nikkei Asia
Paul Ehrlich Institut News Start Of The Rolling Review Procedure For Sinovac S Whole Virus Vaccine Called Covid 19 Vero Cell Inactivated At The European Medicines Agency

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Egypt Reports No Serious Side Effects On Chinese Covid 19 Vaccine Cgtn

Sinovac Reports Positive Data From Phase I Ii Trials Of Coronavac

Vero Cell News Articles Etc European Pharmaceutical Review

Sinopharm Bibp Covid 19 Vaccine Wikipedia

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Who Approves China S Sinopharm Covid Vaccine For Emergency Use

Taiwan Puts All Eggs In One Basket By Refusing Mainland Vaccines Global Times
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
World S First Covid 19 Vaccine Factory Qualified For Mass Production Cgtn

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says